
Small amounts of contaminants, big impact on the pH
Recently, there has been increased focus and attention on the amount of metal ion leaching, for drinking water applications in particular. This was the reason for the investigation into the metal ion leaching behaviour of different brazing filler metals used for drinking water, which was presented at the International Brazing and Soldering Conference (IBSC) in 2021.
Following that presentation, a number of questions have come up as to how metal ion leaching behaviour could differ for applications using higher purity water such as deionised (DI) water or ultra-pure water (UPW). Purified water is used for an increasing number of applications that are relevant for brazed components and higher purity waters are generally more corrosive to metals compared to drinking water, as the tendency for passivation of the metallic surfaces by protective oxide or scale formation is decreased. As higher purity water has limited or no buffering capabilities, small amounts of contaminants (e.g. ions or dissolved gases) can have a big impact on the pH.
With that in mind, a recent investigation was carried out by Torstein Gröstad and Marcus Persson where the metal ion leaching of AISI 316L components brazed with different filler metals, Cu-foil (99,9% Cu), BNi-5, BNi-12, BrazeLet® Ni613, BrazeLet F86 and a Ni-Cr-P-Si-B foil, was investigated and compared to suggest the best choice for minimising the amount of metal ion leaching.
Lowest amount of leaching found in BrazeLet F86 and BrazeLet Ni613
Purified water is used for an increasing number of applications that are relevant for brazed components, such as lasers, medical equipment, laboratory instrumentation, pharmaceutical production, food processing and semiconductor manufacturing. In many of these applications, accurate temperature control of the purified water is necessary, achieved by the use of brazed plate heat exchangers. There are also a number of applications where purified water is used for cooling applications, in particular for electrical applications to prevent short circuits. Examples include cooling of induction furnaces, high-power rectifiers for DC-smelters and converters.
In the case of brazed components, such as brazed plate heat exchangers, the leaching of metal ions comes from the interaction of both the base material and the brazing filler metal with the media that passes through it.
The recent investigation found that the metal ions that leached most significantly into the ultra-pure water were Cu, Fe, Mn, and Ni.
Among the brazing filler metals included in the investigation, the ones that experienced the lowest amounts of metal ion leaching were BrazeLet F86 and BrazeLet Ni613. BNi-5 and BNi-12 resulted in moderate metal ion leaching and in some cases, corrosion attacks visible to the naked eye in the brazed joint fillets. By far the most extensive metal ion leaching was observed for the two reference samples with Cu foil and the Ni-Cr-P-Si-B foil.
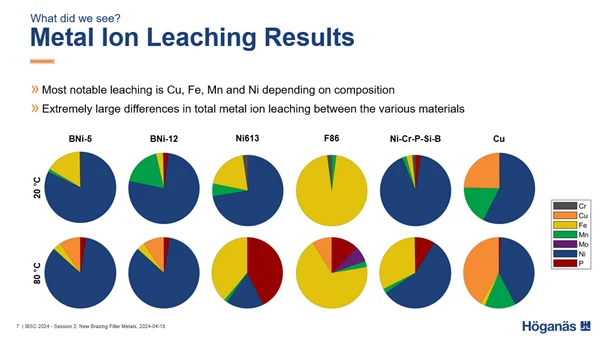
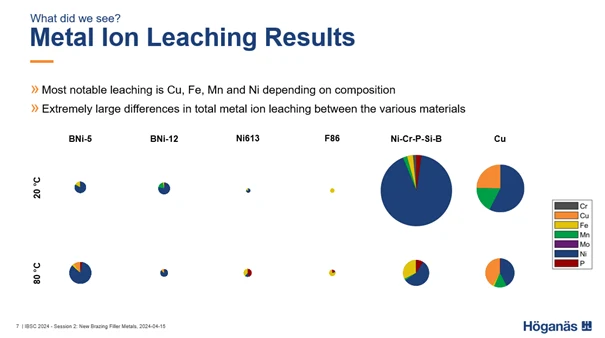
This research underscores the importance of selecting appropriate brazing filler metals to ensure the purity and effectiveness of ultra-pure water systems. Want to know more about choosing the right brazing filler metal?
#InsightsByHoganas
